PEP07: A novel, brain penetrant oral CHK1 inhibitor for the treatment of AML and MCL (6th Annual DDR Inhibitors Summit 2023)
Bettice Chen, Kyla Grimshaw, Jack Cheng, Allen Lee, Mel Liu, Meriel Major, Bob Boyle, Hong-Ren Wang

In November 2020, PharmaEngine and UK-based Sentinel Oncology entered into an exclusive collaboration and license agreement for SOL-578 (PharmaEngine later renamed PEP07), a CHK1 inhibitor. The partnership includes cooperating R&D of GLP toxicology and IND enabling studies of PEP07. In September 2022, PharmaEngine exercised the option for a Worldwide Exclusive License Agreement for PEP07 from Sentinel Oncology.
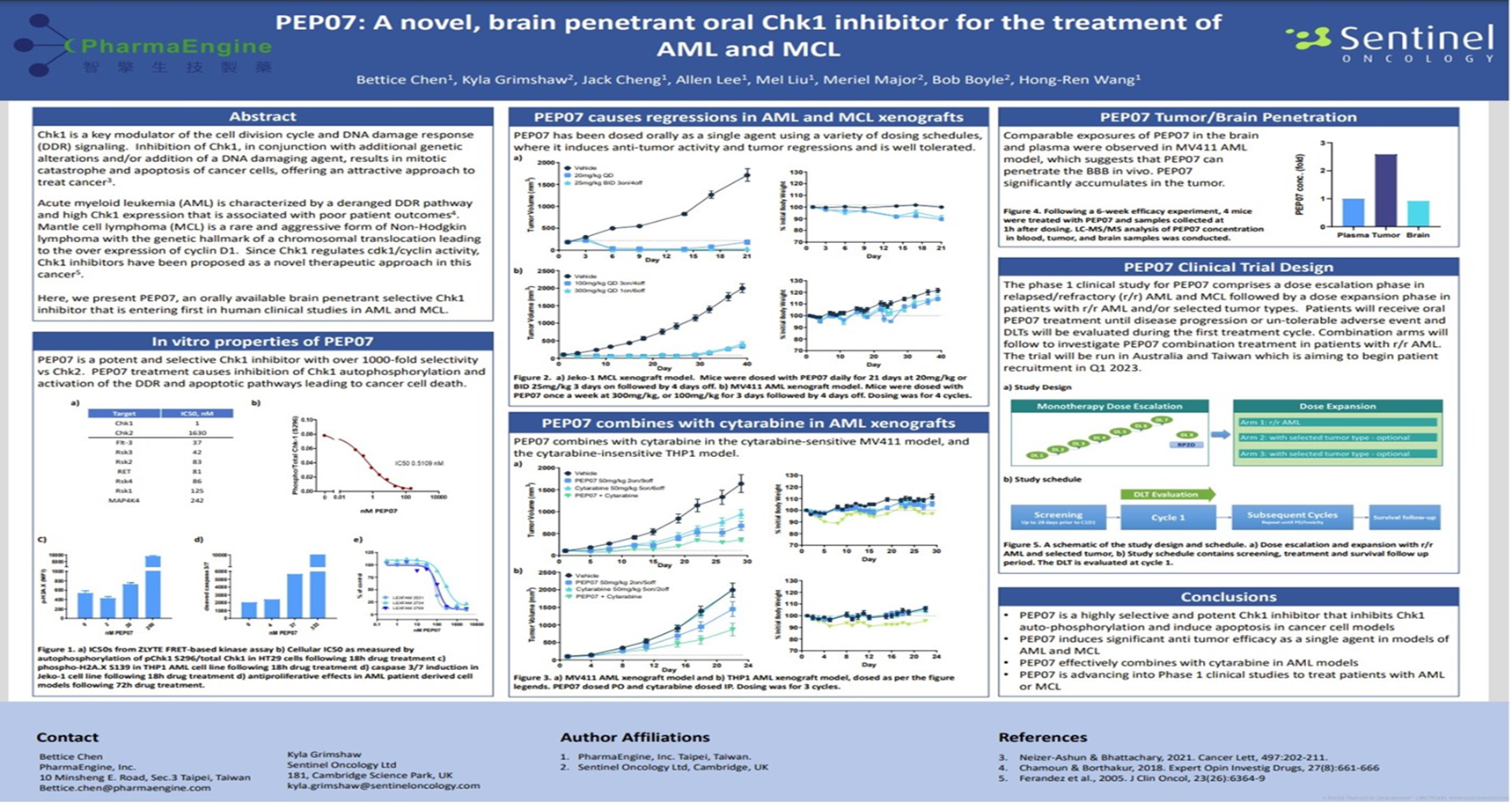
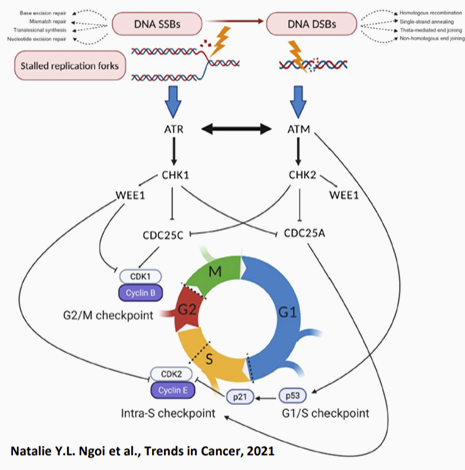
CHK1 is the main regulator of DNA damage and replication checkpoints in cells. It plays a key role in regulating the cell cycle and in DNA damage repair, and can affect cell survival and apoptosis.
CHK1 has been found to be highly expressed in a variety of tumors, including hematological tumors and solid tumors. Moreover, tumor cells with high expression of CHK1 are more resistant to DNA damage reactions caused by radiotherapy, chemotherapy or other tumor treatments, promoting the generation of more malignant tumor cells, and can lead to the occurrence of drug resistance and tumor recurrence.
PEP07 is a potentially the best-in-class among all CHK1 medicines with features such as high kinase selectivity, brain penetrating and oral bioavailability. The main function is to trigger replication catastrophe which leads to apoptosis in the cancer cell during DNA damage. PEP07 has demonstrated significant single-agent activity in inhibiting cancer cell growth and shows R&D potential in oncology therapy.
.png)

PharmaEngine announces first patient dosed in Phase I trial of PEP07 for solid tumor cancers
PharmaEngine announces TFDA approval of PEP07 Phase I clinical trial for solid tumor cancers
PharmaEngine announces first patient dosed in Phase I trial of PEP07 for hematological cancers
Bettice Chen, Kyla Grimshaw, Jack Cheng, Allen Lee, Mel Liu, Meriel Major, Bob Boyle, Hong-Ren Wang

